On March 11, 2020, the World Health Organization declared that the coronavirus 2019 (COVID-19) outbreak constitutes a global pandemic. At present, both domestic and foreign authorities are paying close attention to related diagnosis and treatment research and development. COVID-19 related clinical trials currently registered on the website of the US Clinical Trials Database are used as the source of information for this article. The disclosed drugs are listed based on their original clinical uses, drug permit license status and relevant patent information in Taiwan.
The listed drugs can be divided into the three categories according to the status of their patents:
Category 1: There is basically no patent protection; most are old drugs that have been used clinically for many years, and a small number are drugs that have related patents in foreign countries but have not applied for patents in Taiwan.
Category 2: There is no core patent (compound, antibody molecule) of the main active ingredient of the drug in Taiwan, but there are still related patents for specific salts, related composition preparations, uses or preparations of the active ingredient in Taiwan.
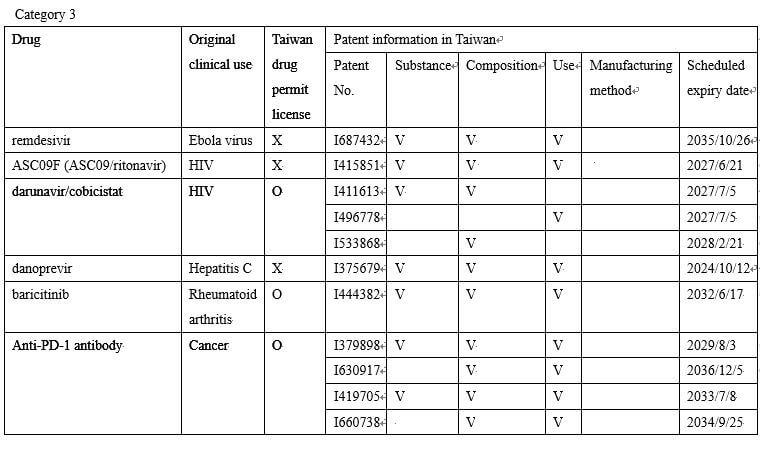
Category 3: There are still core patents protecting the main active ingredients of these drugs in Taiwan.
According to the provisions of Article 59, Paragraph 1, Item 2 of the Patent Act, the effects of an invention patent right shall not extend to necessary acts to exploit the invention for research or experimental purpose(s). Therefore, laboratory experiments do not constitute an infringement.
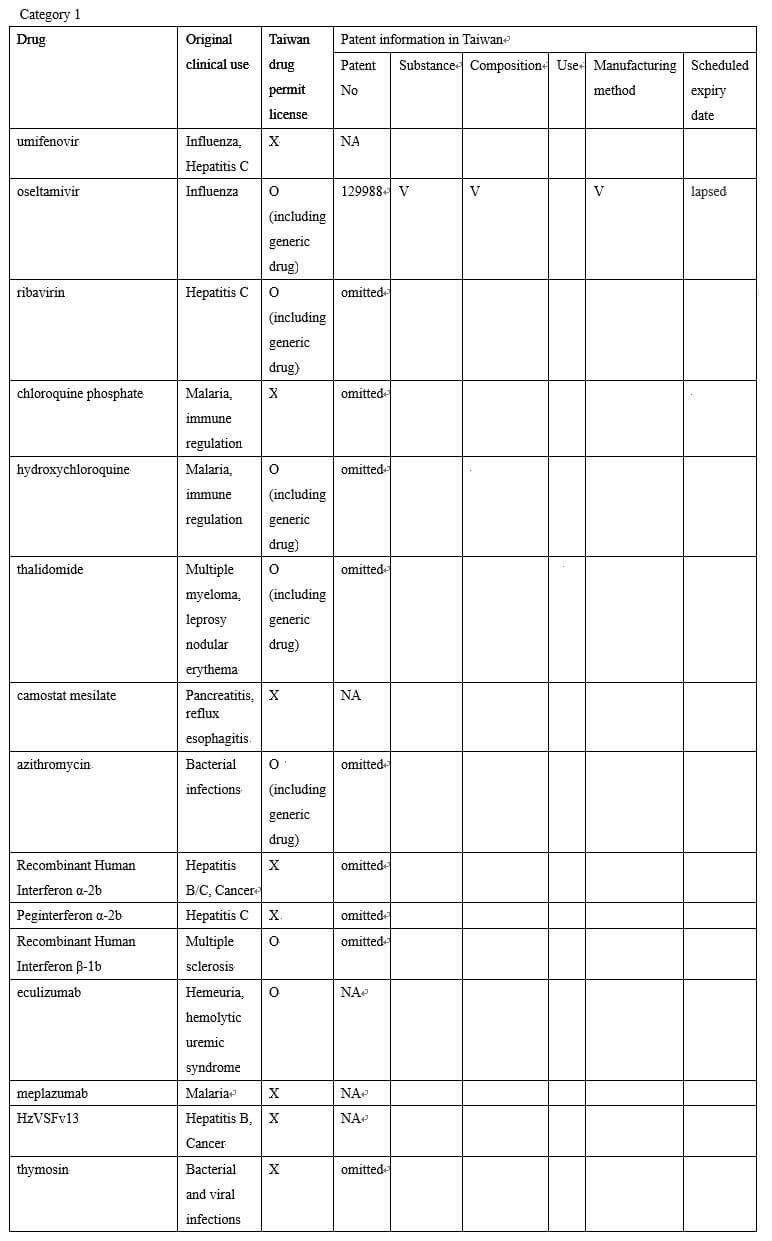

(Translated from the Hot News announced on the website of the Taiwan Intellectual Property Office)
The listed drugs can be divided into the three categories according to the status of their patents:
Category 1: There is basically no patent protection; most are old drugs that have been used clinically for many years, and a small number are drugs that have related patents in foreign countries but have not applied for patents in Taiwan.
Category 2: There is no core patent (compound, antibody molecule) of the main active ingredient of the drug in Taiwan, but there are still related patents for specific salts, related composition preparations, uses or preparations of the active ingredient in Taiwan.
Category 3: There are still core patents protecting the main active ingredients of these drugs in Taiwan.
According to the provisions of Article 59, Paragraph 1, Item 2 of the Patent Act, the effects of an invention patent right shall not extend to necessary acts to exploit the invention for research or experimental purpose(s). Therefore, laboratory experiments do not constitute an infringement.



(Translated from the Hot News announced on the website of the Taiwan Intellectual Property Office)







